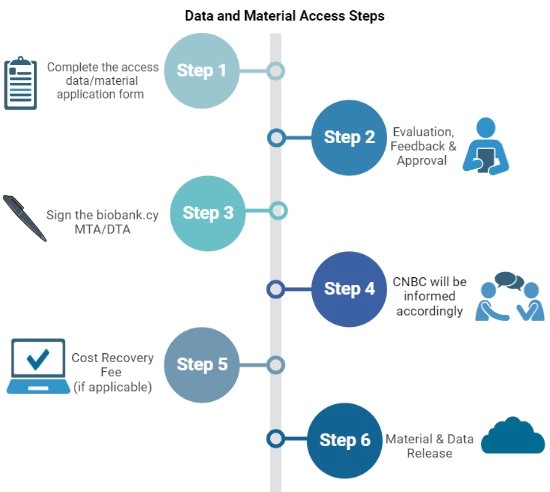
Step 1
Complete the access data/material application form
Complete the access data/material application form (see bellow) including the contact information for a signatory who is authorized to sign the material and data transfer agreement (MTA/DTA) on behalf of your organization. This must be someone other than the PI/Lead Collaborator for each institute.
Step 2
Evaluation, Feedback & Approval
The biobank.cy team will evaluate your application and provide feedback. Upon the approval of your application, you will proceed to step 3.
Step 3
Sign the biobank.cy MTA/DTA
The authorized person from your organization must sign the biobank.cy MTA/DTA.
Step 4
CNBC will be informed accordingly
You will need to transfer an access fee based on your application/request.
Step 5
Cost Recovery Fee (if applicable)
The Cyprus National Bioethics Committee (CNBC) will be informed accordingly.
Step 6
Material & Data Release
Following payment of the access fee, the biobank.cy team will inform you when the material is dispatched/shipped, and the data are available to access.